NUFLOR®
(florfenicol)
Product Description
NUFLOR (florfenicol) Injectable Solution controls all three major bacterial causes of bovine respiratory disease (BRD) and even treats foot rot. Unique, fast-acting, long-lasting antibiotic for treatment of bovine respiratory disease, foot rot and control of respiratory disease in cattle at high risk of developing bovine respiratory disease.

Indications
NUFLOR Injectable Solution is indicated for treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, and for the treatment of bovine interdigital phlegmon (foot rot, acute interdigital necrobacillosis, infectious pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus. Also, it is indicated for the control of respiratory disease in cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni.
Diseases
- Bovine respiratory disease (BRD)
- Histophilus somni
- Mannheimia haemolytica
- Pasteurella multocida
- Bovine interdigital phlegmon
Features And Benefits
- Kills two major bacteria that cause BRD: Mannheimia haemolytica and Histophilus somni. Begins killing within 30 minutes and eliminates 99.9% of those bacteria within 24 hours. Continues to kill for 68 hours and remains inhibitory through 96 hours.1
- Inhibits the third major bacterial cause of BRD: Pasteurella multocida.2
- Effective, versatile, broad spectrum therapy in the hospital and on arrival of cattle at risk of developing BRD.
- Lowers risk with high-risk cattle – treat on arrival.
- Fewer repulls and retreatments.
- Easy to use, requires no mixing or reformulation and isn’t contraindicated in automatic injection equipment.
Administration
For treatment of bovine respiratory disease (BRD) and bovine interdigital phlegmon (foot rot):
NUFLOR Injectable Solution should be administered by intramuscular injection to cattle at a dose rate of 20 mg/kg body weight (3 mL/100 lbs). A second dose should be administered 48 hours later. Alternatively, NUFLOR Injectable Solution can be administered by a single subcutaneous (SC) injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.
NOTE: Intramuscular injection may result in local tissue reaction which persists beyond 28 days. This may result in trim loss of edible tissue at slaughter. Tissue reaction at injection sites other than the neck is likely to be more severe.
For control of respiratory disease in cattle at high-risk of developing BRD:
NUFLOR Injectable Solution should be administered by a single subcutaneous injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.
NUFLOR Injectable Solution DOSAGE GUIDE
| ANIMAL WEIGHT (lbs) | IM NUFLOR DOSAGE 3.0 mL/100 lb Body Weight (mL) | SC NUFLOR DOSAGE 6.0 mL/100 lb Body Weight (mL) |
|---|---|---|
| 100 | 3.0 | 6.0 |
| 200 | 6.0 | 12.0 |
| 300 | 9.0 | 18.0 |
| 400 | 12.0 | 24.0 |
| 500 | 15.0 | 30.0 |
| 600 | 18.0 | 36.0 |
| 700 | 21.0 | 42.0 |
| 800 | 24.0 | 48.0 |
| 900 | 27.0 | 54.0 |
| 1000 | 30.0 | 60.0 |
Recommended Injection Location
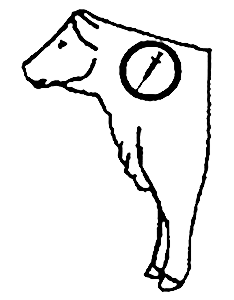
Do not inject more than 10 mL per injection site.
Clinical improvement should be evident in most treated subjects within 24 hours of initiation of treatment. If a positive response is not noted within 72 hours of initiation of treatment, the diagnosis should be re-evaluated.
Supplied
100 mL glass sterile multiple-dose vials.
250 mL glass sterile multiple-dose vials.
500 mL glass sterile multiple-dose vials.
Storage
Store between 2°-30°C (36°-86°F). Refrigeration is not required. Protect from light when not in use. Use within 30 days of first puncture. For the 100mL vials, puncture the stopper a maximum of 3 times. For the 250mL and 500mL vials, puncture the stopper a maximum of 17 times.
Related Products
Learn how you can see a difference in just one dose with Resflor Gold (florfenicol and flunixin meglumine), combining the benefits of Nuflor with the fever-reducing action of Banamine (flunixin meglumine injection).
User Safety Warnings
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains materials that can be irritating to skin and eyes. Avoid direct contact with skin, eyes, and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. Reproductive and developmental toxicities have been reported in laboratory animals following high, repeated exposures to N-methyl-2-pyrrolidone (NMP). Pregnant women should wear gloves and exercise caution or avoid handling this product. The Safety Data Sheet (SDS) contains more detailed occupational safety information.
Precautions
Not for use in animals intended for breeding purposes. The effects of florfenicol on bovine reproductive performance, pregnancy, and lactation have not been determined. Toxicity studies in dogs, rats, and mice have associated the use of florfenicol with testicular degeneration and atrophy. Intramuscular injection may result in local tissue reaction which persists beyond 28 days. This may result in trim loss of edible tissue at slaughter. Tissue reaction at injection sites other than the neck is likely to be more severe.
Contraindications
Do not use in animals that have shown hypersensitivity to florfenicol.
Adverse Reactions
Inappetence, decreased water consumption, or diarrhea may occur transiently following treatment.
Important Safety Information
Do not use in animals that have shown hypersensitivity to florfenicol or animals intended for breeding purposes. Transient inappetence, diarrhea, decreased water consumption, injection site swelling, anaphylaxis and collapse have been associated with the use of florfenicol in cattle. Do not use in calves to be processed for veal or in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. Animals intended for human consumption must not be slaughtered within 28 days of the last intramuscular treatment or 38 days of subcutaneous treatment. A withdrawal period has not been established in pre-ruminating calves. Avoid direct contact with skin, eyes, and clothing. Pregnant women should wear gloves and exercise caution or avoid handling this product. For complete information, see the product package insert.
Contact
U.S. only: Merck Animal Health livestocktechsrvc@merck-animal-health.com or call 1-800-211-3573
For more information regarding efficacy and safety data, go to productdata.aphis.usda.gov
For additional information, please see the product label.
References
1. Varma, KJ, Lockwood PW, Cosgrove MS, Rogers ER, Pharmacology, Safety and Clinical Efficacy of Nuflor (florfenicol) Following Subcutaneous Administration to Cattle. Preceedings of a Symposium Held in Conjunction with the XX World Buiatrics Congress. Sydney, Australia. July 1998: 13-19.
2. Data on file, Merck Animal Health.

Get the latest updates! Sign up to receive cattle health management insights, industry news and more sent straight to your inbox.

