
Highy effective and safe for even the most stubborn cases
A strong, yet gentle solution for severe cases of otitis externa in dogs.

Indications
Posatex® Otic Suspension is indicated for the treatment of otitis externa in dogs associated with susceptible strains of yeast (Malassezia pachydermatis) and bacteria (coagulase-positive staphylococci, Pseudomonas aeruginosa, and Enterococcus faecalis).
Supplied
POSATEX Otic Suspension is available in 7.5 g, 15 g, and 30 g plastic bottles.
Features & Benefits
Triple-action, once-a-day treatment for 7 days for even the most stubborn cases associated with susceptible strains of yeast and bacteria in canines.
ANTIBACTERIAL EFFECT
Orbifloxacin
Effective against a wide variety of Gram-negative and Gram-positive bacteria, including:1 Pseudomonas aeruginosa, coagulase-positive staphylococci, and Enterococcus faecalis
ANTI-INFLAMMATORY ACTION
Mometasone furoate monohydrate
An effective corticosteroid that rapidly reduces discomfort, erythema, and swelling1
Rapidly controls inflammation without adrenocortical suppression2
ANTIFUNGAL ACTIVITY
Posaconazole
Highly effective and safe for even the most stubborn cases
Provides potent, broad-spectrum efficacy, including against Malassezia pachydermatis1
VETERINARY CLINICAL IMPRESSION STUDY3
POSATEX gets strong performance scores from veterinarians, even on the toughest, most chronic cases of otitis externa.
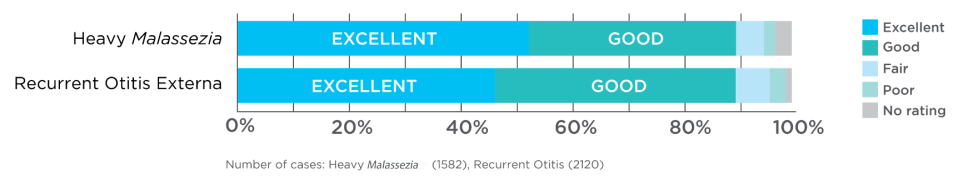
Results
- POSATEX treatment success rated as excellent in 50% OF ALL CASES.3
- In comparison, veterinarians gave prior treatment an “excellent” score in only 10% of all cases.
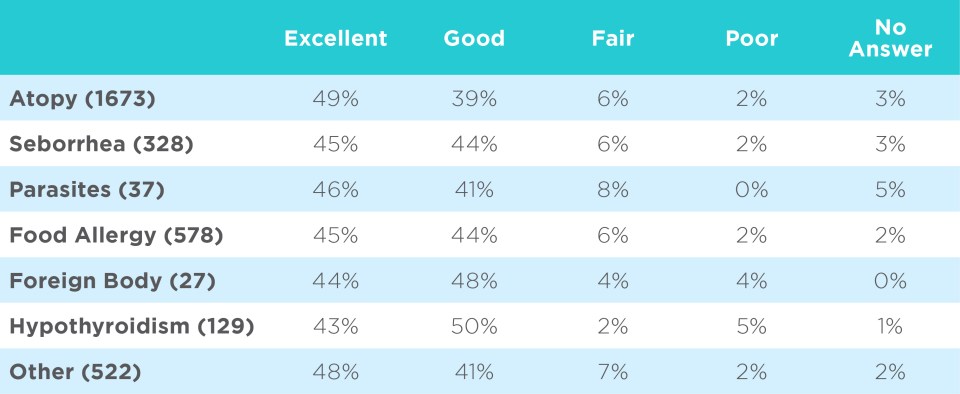
Performance is consistent regardless of primary factors.3
Treat otitis externa the first time.
Veterinarians use POSATEX most often to treat recurrent otitis externa and cases with heavy
Malassezia infection, and they report excellent treatment results with Posatex.3
Diseases/Parasites
Description
Each gram of POSATEX Otic Suspension contains 10 mg of orbifloxacin; mometasone furoate monohydrate equivalent to 1 mg mometasone furoate; and 1 mg of posaconazole in a mineral oil-based system containing a plasticized hydrocarbon gel.
Administration and Dosage
- Adminster once daily for 7 days — Helps ensure pet parent compliance to treatment
- 4 drops per ear for dogs less than 30 lbs
- 8 drops per ear for dogs equal to or greater than 30 lbs
IMPORTANT SAFETY INFORMATION
Posatex® is contraindicated in dogs with known or suspected hypersensitivity to quinolones, mometasone furoate monohydrate, or posaconazole. Do not use in dogs with known tympanic perforation. The safe use in dogs used for breeding purposes, during pregnancy or in lactating bitches, has not been evaluated.
For additional information, please see the product label.
References
- Posatex® Otic Suspension [Orbifloxacin, Mometasone Furoate Monohydrate and Posaconazole] [Product Information]. Madison, NJ: Intervet Inc.; 2019.
- Reeder CJ, Griffin CE, Polissar NL, et al. Comparative adrenocortical suppression in dogs with otitis externa following topical otic administration of four different corticosteroid-containing medications. Vet Ther. 2008;9:111-121.
- Data on file, Merck Animal Health.
