INCURIN® TABLETS
(estriol)
A treatment for female canine urinary incontinence that contains estriol, a naturally occurring, short-acting estrogen found in female dogs with the convenience of once-a-day dosing.

Overview
For the control of estrogen-responsive urinary incontinence in ovariohysterectomized female dogs
Features and Benefits
- Proven effective in reducing or eliminating urinary incontinence1
- 93% of dogs treated were improved or continent by 6 weeks1
- Convenient, once-a-day dosing
- One simple starting dose for all female dogs and just 1 tablet size to stock
- Titrated to the lowest effective dose
Important Safety Information
- Well tolerated in a long-term field study (42-month) involving 324 client-owned ovariohysterectomized female dogs.1,3
- Safely used worldwide for more than 16 years
- Short receptor binding time limits estrogen-related side effects.1,2,4
Dosage and Administration
- Tablets administered orally
- All dogs start out with a once-daily dose of two 1 mg Incurin tablets regardless of body weight
- Titrated to the lowest effective dose
Supplied
12x blister (30 x 1 mg)
For further information including dose rate and complete directions and warnings, please see the Product Label by clicking here
Fair Balance
Incurin is indicated for the control of estrogen-responsive urinary incontinence in ovariohysterectomized dogs. The most common side effects associated with Incurin treatment under field conditions included loss of appetite, vomiting, excessive water drinking, and swollen vulva. The safety and effectiveness of Incurin Tablets have not been evaluated in dogs less than 1 year of age, intact female dogs, male dogs, dogs used for breeding, or lactating dogs. Incurin is contraindicated in dogs showing polyuria secondary to polydipsia, or in pregnant dogs. Please see the product label for full prescribing information.
For additional information, please see the product label.
References
1. Incurin (estriol) Tablets for Dogs [Freedom of Information Summary]. Madison, NJ: Intervet Inc.; 2011.
2. Clark JH, Hardin JW, McCormack SA. Mechanism of estrogen agonists and antagonists. J Anim Sci. 1979;49:46–65.
3. Data on file, Merck Animal Health.
4. Clark JH, Markaverich BM. The agonistic and antagonistic actions of estriol. J Steroid Biochem. 1984;20(4B):1005–1013.1.
Efficacy And Safety
Incurin (estriol) Tablets have been proven to reduce or eliminate urinary incontinence in spayed female dogs.1
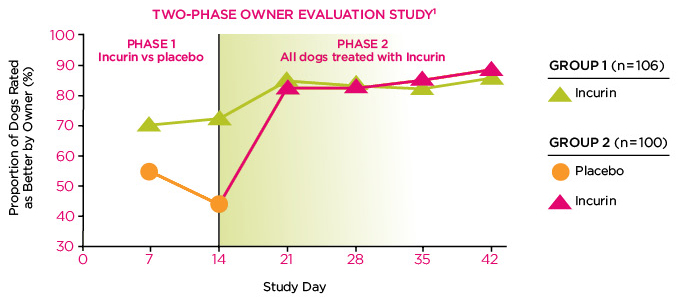
Incurin helps restore urinary continence—The majority of owners participating in a study of over 200 subjects felt their dogs improved after being treated with Incurin.1
- 93% of dogs treated with Incurin were improved or continent by 6 weeks1
- 51% of dogs were titrated down to doses less than 2 mg daily; of these, 99% were improved or continent by 6 weeks1

Safety Studies
In a target animal study, there was no evidence of bone marrow suppression at 5 times the maximum label dose (10 mg daily) for 6 months. There were no estriol-related changes on bone marrow smears evaluated microscopically from dogs in the 10 mg (5X) group. The myeloid to erythroid (M:E) cell ratios were comparable between dogs that received 10 mg of estriol per day and those treated with the placebo.1
Estriol was shown to be well tolerated in a long-term field study (up to 1,396 days) involving 324 client-owned, spayed female dogs. Based on the relatively low frequency and mildness of the adverse events and their level of association with estriol, no safety concerns associated with longer-term use of the product were observed.1,2
Estriol has been safely used worldwide in dogs for more than 16 years. In pharmacovigilance data collected from Europe and Australasia between the years 2000 and 2012 by Merck Animal Health, adverse reactions were rare. Of reported reactions, vulvar swelling, attractiveness to male dogs, gastrointestinal signs, and localized or generalized hormonal-pattern alopecia were the most common observations.1,3
Incurin is indicated for the control of estrogen-responsive urinary incontinence in ovariohysterectomized dogs. The most common side effects associated with Incurin treatment under field conditions included loss of appetite, vomiting, excessive water drinking, and swollen vulva. The safety and effectiveness of Incurin Tablets have not been evaluated in dogs less than 1 year of age, intact female dogs, male dogs, dogs used for breeding, or lactating dogs. Incurin is contraindicated in dogs showing polyuria secondary to polydipsia, or in pregnant dogs.
For further information, including complete directions and warnings, please see the full prescribing information.
References:
1. Incurin™ (estriol) Tablets for Dogs [Freedom of Information Summary]. Madison, NJ: Intervet Inc.; 2011.
2. Data on file, Merck Animal Health.
3. Incurin™ (estriol) Tablets for Dogs [package insert]. Madison, NJ: Intervet Inc.; 2011.
Dosing And Administration
Incurin (estriol) Tablets are easy to dose.

Safety Studies
The dose of Incurin is not dependent upon body weight. All dogs should receive an initial dose of 2 mg (two 1-mg tablets) orally once per day for a minimum of 14 days.
After urinary incontinence is controlled, the lowest effective daily dose of Incurin should be determined by decreasing the dose in a step-wise manner from 2 mg once daily (2 tablets) to 1 mg once daily (1 tablet), then 0.5 mg once daily (1/2 tablet) depending upon the response of the individual dog. There should be a minimum of 7 days between each dose adjustment. After the lowest daily dose that controls urinary incontinence is identified, the dose may be decreased further by administering once every 2 days.
Dogs should not receive more than 2 mg of Incurin per day (2 tablets). If the dog does not respond to 2 mg per day, the diagnosis should be re-assessed.
Incurin is indicated for the control of estrogen-responsive urinary incontinence in ovariohysterectomized dogs. The most common side effects associated with Incurin treatment under field conditions included loss of appetite, vomiting, excessive water drinking, and swollen vulva. The safety and effectiveness of Incurin Tablets have not been evaluated in dogs less than 1 year of age, intact female dogs, male dogs, dogs used for breeding, or lactating dogs. Incurin is contraindicated in dogs showing polyuria secondary to polydipsia, or in pregnant dogs.
For further information, including complete directions and warnings, please see the full prescribing information.
